Quality
Outside and Inside!
All HGH Card & Care Service products are high-quality products. You can see and feel that! Nevertheless, as a customer, you can influence the quality and value in terms of the cardboard, the finishing and, above all, the content.
Printing is carried out in an FSC-certified and climate-neutral production facility! As standard, the cards are printed on wood-free, white solid chromosilicate cardboard 240 g/m² and finished with a protective coating. However, you can also order special cardboard for your PflasterCard®. The range extends from environmentally friendly paper to high-quality fine papers with special effects such as “metallic.” On request, the PflasterCard® can also be cellophane-wrapped.
For the 4-sided, 6-sided and 8-sided credit card-sized product variants, the double adhesive strips are either attached with silicone adhesive dots or inserted into a crystal-clear, plasticizer-free foil bag, which is first manually glued to the lower inside of the folding card.
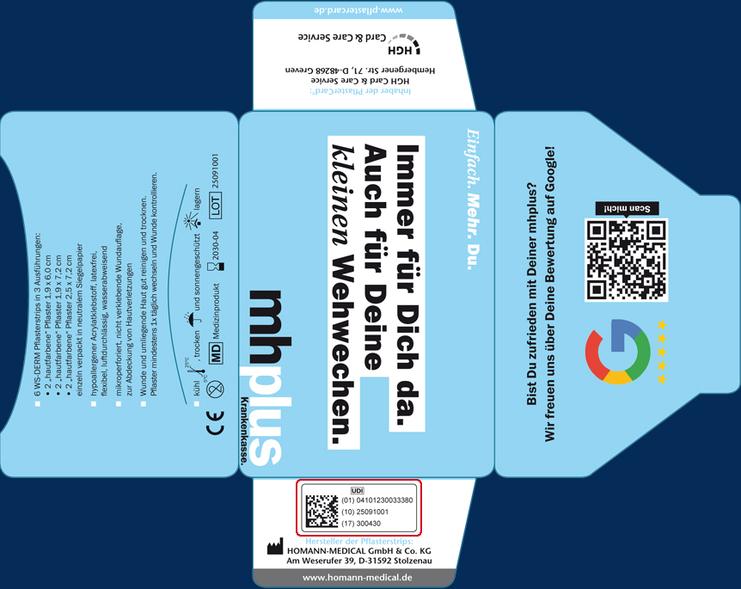
The value of the cards depends not least on the finishing options you have chosed and the selected content. A plaster is a Class I medical device in accordance with the EU Medical Devices Regulation (MDR) 2017/745 and UDI 2025. The HGH Card & Care Service takes this very seriously. Therefore, the following information is printed on every PflasterCard®:
Information about the respective content:
- manufacturer of the plasters
- number of plasters
- plaster dimensions
- type of plasters
- product characteristics
Legal labeling:
- LOT no.
- expiration date
- single use
- CE mark
- MD logo
- UDI logo
- manufacturer's information
Storage instructions:
- cool
- dry
- protected from sunlight
Instructions for use and handling:
- to cover skin injuries
- clean the wound and surrounding skin thoroughly
- change the plaster at least once a day
The particularly important and detailed information on product properties and material composition:
- sealing paper
- carrier material
- wound dressing
- adhesive compound
can be found under the menu item “Pflasterauswahl” for the individual products of the respective manufacturer. All plasters from German manufacturers are usually produced as double strips and have a shelf life of 5 years.
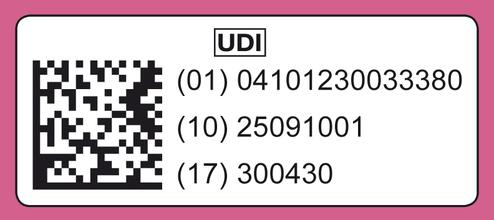
The European Medical Device Regulation (MDR) 2017/745 requires manufacturers of Class I medical devices to affix a machine-readable UDI code (UDI Unique Device Identification) to medical device packaging from May 2025 onwards, indicating the manufacturer, item number, LOT number, and expiration date. This global system for uniform product identification for medical devices was developed in the USA by the FDA (Food and Drug Administration). It enables clear and unambiguous identification of specific products on the market and facilitates their traceability. All information flows into the UDI database, which is part of the European medical device database EUDAMED.
The European Medical Device Regulation (MDR) 2017/745 requires manufacturers of Class I medical devices to affix a machine-readable UDI code (UDI Unique Device Identification) to medical device packaging from May 2025 onwards, indicating the manufacturer, item number, LOT number, and expiration date. This global system for uniform product identification for medical devices was developed in the USA by the FDA (Food and Drug Administration). It enables clear and unambiguous identification of specific products on the market and facilitates their traceability. All information flows into the UDI database, which is part of the European medical device database EUDAMED.
You can choose your preferred plaster from the plaster strip selection in the gallery. No other advertising company offers you this professional and transparent presentation of plaster selection, information on contents, legal labeling, product properties, and material composition!